Observations of a Possible Biological Alteration of the Radioactive Decay
Of Thorium by Fungi
N.A. Reiter
Dr. S.P. Faile
12 August, 2002
Abstract:
Previous work on the problem of anomalous biological transmutation, carried out by Kervran, et al. has dealt primarily with lighter elements, such as calcium and potassium. Little appears in literature concerning the possibility that biological systems could transmute or otherwise remediate heavy nuclear materials undergoing radioactive decay. Results of a simple experiment conducted in August of 2002 by us suggest that the radioactive decay of thorium was altered dramatically by fungal growth in an organic matrix to which the thorium had been added in the form of the nitrate. Results of the experiment, and theoretical implications are discussed.
Background and Objective:
In spring of 2002, observations were made of an unkown fungus that had taken hold in the residence and work area of Dr. Faile. Samples were submitted to a local medical center and were in turn sent out for analysis. A positive match was not made, however the samples were generally identified as being ascomycete gyromitra - like. By historical tracking, deductive reasoning, and intentional culturing, Dr. Faile concluded that the fungus in question had likely originated at a location in southern Ohio of archaeological interest, the Fort Hill earthworks.
In one sense, while the appearance of the fungus in Failes residence was a surprise, the overall character was not terribly unusual. It was found that a preferred culture medium was a blend of plain soy milk and common wheat puff cereal.
While examining some of the cultures in process, we wondered if the novel fungus might have some additional ability to display some traits we had long hypothesized might exist biological transmutation and the ability of fungi to influence the process of radioactive decay. Thus the availability of the cultures present under Dr. Failes supervision provided a good opportunity to test this notion.
In late July, Dr. Faile provided a sizeable amount of the dried fungus to NR for testing.
Procedure:
We procured from SPF a bag of semi-dried fungal matrix from his experiments in Cincinnati. For detection of radiation, we use our Baird Atomic 914 lab Geiger counter. With voltage set for 900V, we confirm that the background count rate in the lab appeared to typically run between 10 and 30 CPM.
Our experiment was performed at our lab location in the Toledo, Ohio area. The ambient consisted of a typical air conditioned office / lab atmosphere, with typical temperature running about 24oC +/- 2oC.
Two 6 inch square plastic tubs were washed thoroughly with methanol, then dried. Room temperature unflavored soy milk was added to each, 150 ml worth. To the first tub, our control, an additional 20 ml of soy milk was added, along with 20 ml of a saturated solution of thorium nitrate in distilled water.
To the second tub, we add 20 ml of soy milk in which 1 gram of our semi-dried fungal matrix was minced and mixed. We then add 20 ml of the thorium nitrate:H2O solution.
Both the control and cultured tubs were shaken to disperse the solutions. We note that within a few seconds, the thorium nitrate caused a partial curdling of the soy milk.
The Geiger counter was used to take count rates for both tubs, beginning immediately after preparation, and then periodically for the next 168 hours. This data was recorded, and is presented herein. Our readings were taken by holding the GM tube vertically over the soymilk solutions, at tub center and at tub corners. Max and min count rates were recorded. We also record our visual records of the physical properties for both cultured and control tubs.
Results:
|
Elapsed Time (Hrs.) |
Tub (Cultured / Control) |
Min / Max count rate (CPM) |
Notes (Appearance) |
|
0 |
Cultured |
180 220 |
|
|
0 |
Control |
180 220 |
|
|
3 |
Cultured |
240 260 |
|
|
3 |
Control |
180 220 |
|
|
8 |
Cultured |
260 280 |
|
|
8 |
Control |
180 220 |
|
|
24 |
Cultured |
300 500 |
Lt. Yellow soft mat forming at center region |
|
24 |
Control |
200 220 |
|
|
48 |
Cultured |
300 500 |
Entire contents semi-solid |
|
48 |
Control |
180 220 |
Contents still runny |
|
52 |
Cultured |
260 480 |
|
|
52 |
Control |
180 220 |
|
|
72 |
Cultured |
240 340 |
|
|
72 |
Control |
180 260 |
Isolated regions of mold starting to grow |
|
120 |
Cultured |
220 340 |
Crenulated fuzzy green spore producing mat around periphery Center area bare and semi-solid Dead at center? |
|
120 |
Control |
180 - 300 |
|
|
144 |
Cultured |
220 340 |
|
|
144 |
Control |
240 340 |
Assorted moldy regions growing |
|
150 |
Cultured |
240 340 |
|
|
150 |
Control |
240 340 |
|
|
168 |
Cultured |
240 360 |
|
|
168 |
Control |
240 340 |
|
|
168 (Stirred up) |
Cultured |
260 360 |
|
|
168 (Stirred up) |
Control |
280 - 300 |
|

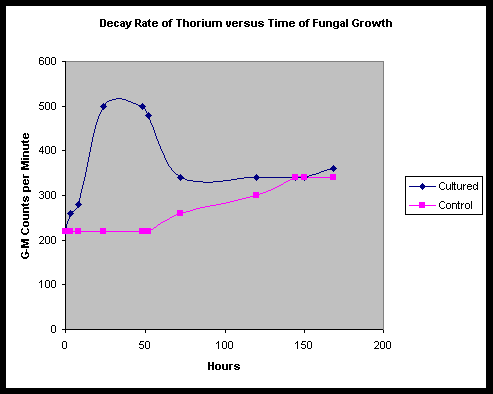
As may be plainly seen, a dramatic increase in the emission rate is noted in the cultured tub, beginning at T+ 3 hours, and rising to a peak level between 24 and 48 hours. From previous observations by Faile, the Fort Hill fungus seems to typically begin to culture and grow rapidly during this time, with a mat forming between 12 and 24 hours at room temperature.
During this same time, we note little change in the emission rate of the control tub. However, by about 72 hours into the testing, we notice isolated patches of mold beginning to form on the still runny curdled surface of the control tub soy milk. This development seems to be accompanied by a steady rise in the emission rate of the control tub.
At a point shortly after T+ 48 hours, we observe the emission count rate for the cultured tub start to decline, seeming to stall out and level off at about 72 hours. We point out that at T+ 120 hours, the control and cultured tubs appear to pass each other, though the control tub appears to stabilize by around 144 hours.
To rule out the possibility of stratification of the thorium near the top of the culture surfaces, at 168 hours, we stir the contents of both tubs, to spread out the contents. Readings are then taken, and we find that the average CPM tends to remain constant, however the lateral deviations seem to diminish in the control tub. In the cultured tub, we find that there still exist highs and lows in CPM, however these seem to be scrambled with the disordering of the fungal mat.
Discussion:
We do not have any conventional explanation for the dramatic results observed. The rise and fall of decay count rates appears totally anomalous, however, we are continuing to evaluate the experiment design for flaws. Thus far, we have found none. The onset of the rise in CPM for both tubs appeared to closely coincide with the period of visible growth of fungi. The cultured tub, containing the greater quantity of fungus, seems to have peaked higher and faster. However, the stabilization after drop-off may have to do with the apparent death of the fungal mass at the tub center (we cannot say at this time why the fungus appeared to die there - high radioactivity or a dramatic change in pH are both plausible). In the case of the control tub, the eventual rise in CPM was slower and less dramatic, identifying with the character of the mold growth therein.
Our speculations at this time would allow two potential explanations of what we have seen - apart from artifact:
We currently (12 August 2002) have a second replication round of tests underway, and plan to continue looking at this unique phenomenon. In this second round, we also have included a fungal culture containing a uranium compound, uranyl acetate. Samples from these cultures will be examined carefully by EDS to look for positive signs of elemental transmutation.
Workable models for biological transmutation do not seem to be prevalent. One concept might invoke the nanoscopic sizes of some bacteriological or fungal components. Could structures of this extremely small size domain (less than .5 microns) manipulate vacuum fluctuations in coherent or useful ways, possibly affecting nuclear decay? Or do fungal life forms produce some unknown form of radiation capable of interacting with heavy nuclei?
It is our hope that further experiments will clarify whether either of these notions, or others are viable.
