Experiments in Biologically Catalyzed Nuclear Alteration:
Fifth Report
18 August, 2003
Nicholas A. Reiter and Dr. Samuel P. Faile
Background:
Throughout 2003, we continued to observe and report on the apparent biological alteration of elemental constituents in an ascomycete culture to which thorium and uranium compounds had been added. Consistently, this phenomenon - whose apparent products we have analyzed by EDS spectroscopy - has been accompanied by anomalous increases and decreases in the rate of radioactive decay of the Th or U. In this summary, we report our further efforts to find patterns and consistency in this phenomenon, as well as making more comparisons between the activity of mushroom-like Fort Hill fungus and baker's yeast. We also describe our attempts to produce a useful amount of transmutative product in the form of certain platinum group metals.
General Protocol Notes:
We have striven to be consistent in our protocols, over the long duration of this project. For radioactive source materials, we have used thorium nitrate and uranyl acetate. We have consistently used one brand of soymilk and yeast. Yeast cultures have been grown in a 250 ml pyrex beaker, while cultures using Fort Hill fungus have generally been started in shallow plastic tubs. Both beakers and tubs are cleaned with acid and DI water before use. During growth, loose lids are placed over media to keep dust out, but allow exchange with atmosphere. All growth experiments were carried out in an air-conditioned lab environment. Before measuring with our Baird Atomic 916 Geiger counter, we stir yeast cultures thoroughly. Beaker culture measurements are made with the GM tube held one inch over the upper surface of the measured material. Shallow tub measurements are made by holding the GM tube one inch above the center of the culture. We have recorded min and max readings, however for our purposes of reporting and plotting, we have used primarily the maximum reading attained, after 60 seconds of sampling.
EDS analysis has been performed using a Jeol SEM and Tracor Northern TN 5400 EDS system with Iridium software and light element detector. We typically have run an accelerating voltage of either 15 or 20kV, unless otherwise noted.
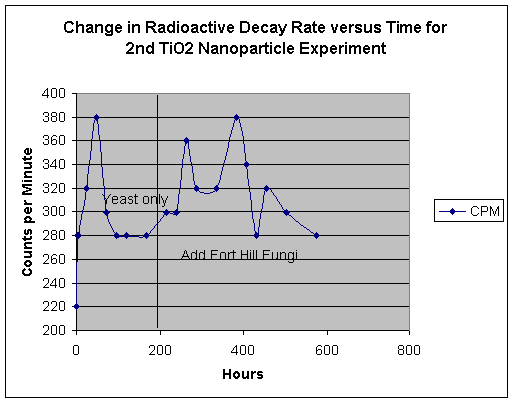
Effects of TiO2 Nano-particle Media - 2nd Run:
Our first attempt to use TiO2 nano-particle media produced an interestingly intense reaction in the earliest stage of yeast growth, a powerful foaming expulsion of culture media from our test beaker. We desired to repeat this experiment, with a much greater level of watchfulness. Thus, our basic recipe was the same as reported at the end of our fourth report, except for the substitution of thorium nitrate for uranyl acetate. As in the first experiment using the nano-particles, we observed a strong foaming action of the contents, but were this time able to control the foaming by stirring. Thus, we were able to conserve the contents of the beaker. See below:
|
Titania Nanoparticle Experiment Replication |
||||||||
|
100 ml soy milk |
||||||||
|
1 tsp sugar |
||||||||
|
25 ml of aqueous suspended TiNano40 |
||||||||
|
20ml of half saturated Thorium Nitrate in H2O |
||||||||
|
1 pack of dried yeast |
||||||||
|
We monitor the appearance of the culture contents carefully! |
||||||||
|
Date |
Hours |
Max CPM |
Comments |
|||||
|
14-Apr |
0 |
220 |
||||||
|
2.5 |
280 |
|||||||
|
4.5 |
280 |
Max foaming |
||||||
|
24 |
320 |
Foam down |
||||||
|
48 |
380 |
Some Stratifying - stir before measuring |
||||||
|
72 |
300 |
|||||||
|
96 |
280 |
Very liquidy |
||||||
|
120 |
280 |
Add Fort Hill fungi |
||||||
|
168 |
280 |
|||||||
|
216 |
300 |
|||||||
|
240 |
300 |
|||||||
|
264 |
360 |
mat starting to gel |
||||||
|
288 |
320 |
re-liquify! |
||||||
|
336 |
320 |
|||||||
|
384 |
380 |
mat re-forming, gel blob near center |
||||||
|
408 |
340 |
re-liquify |
||||||
|
432 |
280 |
|||||||
|
456 |
320 |
liquidy |
||||||
|
504 |
300 |
|||||||
|
576 |
280 |
|||||||
With the addition of Fort Hill fungal stock, we once again see a re-activation of our basic effect. However the phenomenon of repeated mat formation and re-liquification is a surprising event. Note how the rise and fall of emission rates appears to follow the trend to form a fungal mat, which then apparently fails and dissolves.

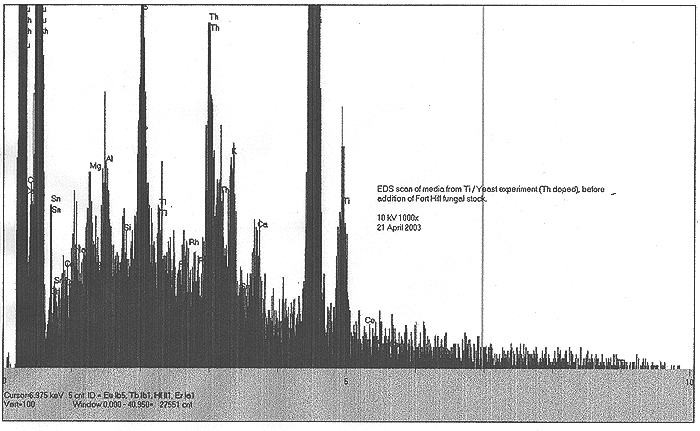
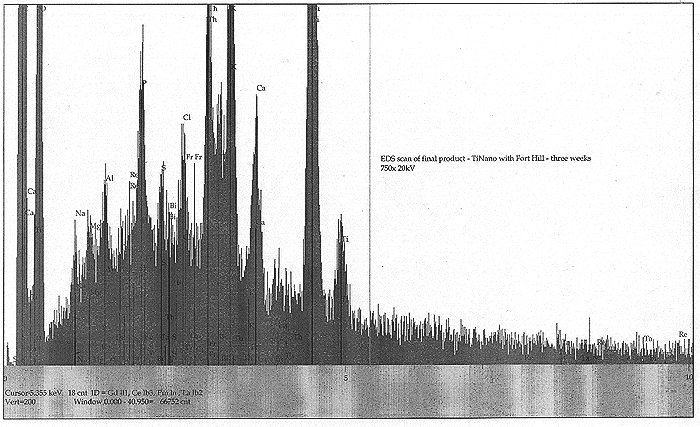
EDS Results:
EDS scans were taken right before the addition of the Fort Hill stock (indicating the effects of yeast only) and also at T+ three weeks, at the end of the experiment (indicating the influence of the Fort Hill fungus). In the first scan, we find small peaks indicating trace amounts (less than 1%) of Pd, Ru, Rh, Ce and Sn. A somewhat larger peak appears to be identified with thallium (Tl). By week number three, the influence of the Fort Hill fungus has seemingly altered the elemental profile! Most of the previous trace elements, including the Tl are gone, however, we now find Bi, Re, Ta, and a possible signature of very short lived and highly exotic Fr. It would appear that as long as fungal activity is present, the final elemental traces to be discovered are in a state of flux.
Exploring the Influence of Boron - Two Experiments:
In his program of qualitative analysis of fungal cultures grown with different additive compounds, SPF considered that boron might constitute an interesting element for examination. Boron has been cited by M.H. Miles et al as being an activeelement in cold fusion processes. Initial cultures of Fort Hill fungus grown at his Cincinnati location showed unusual growth patterns in both soy milk and cow's milk to which a small amount of commercial borax (sodium borate) was added. In one case, an extended arrangement of pores and filaments was observed, unlike any previous culture recipe. Dr. Faile hypothesized that sodium may have been playing a counter- or detrimental role to an innate beneficial effect of boron. Plans were made for NR to grow some yeast cultures using other boron compounds, such as weak boric acid. However, when these were not found to be available, borax was chosen as a default ingredient. Our first experiment was performed like previous yeast culture runs, in a beaker. We then followed up with a Fort Hill fungus - only run, in a shallow tub. An interesting phenomenon was observed, that had not been noticed with earlier runs - though it had not been looked for up to that point. In the early hours of the yeast activity, we find that the beaker became noticeably chilled. This was measured as a dramatic temperature drop of about 8 degrees C! We ponder under what circumstances the reactions of yeast become endothermic. Apart from this, we ultimately found the borax - yeast culture to produce some of the most promising hints of platinum group metals by EDS:
|
1st Boron Enriched Experiment |
||||||||
|
28-May-03 |
||||||||
|
100 ml soy milk in 250 ml beaker |
||||||||
|
20 ml half saturated thorium nitrate:H2O |
||||||||
|
1 tsp. sugar |
||||||||
|
2 grams of commercial borax |
||||||||
|
1 packet of baker's yeast |
||||||||
|
Date |
Hours |
Max CPM |
Comments |
|||||
|
28-May |
0 |
160 |
Very foamy within minutes |
|||||
|
28-May |
3 |
120 |
Beaker tangibly colder - why? |
|||||
|
24 |
120 |
Very watery but yeast still working |
||||||
|
48 |
100 |
|||||||
|
120 |
80 |
|||||||
|
144 |
100 |
|||||||
|
168 |
80 |
|||||||
|
192 |
80 |
|||||||
|
216 |
80 |
add Fort Hill stock |
||||||
|
240 |
100 |
|||||||
|
288 |
120 |
|||||||
|
312 |
160 |
|||||||
|
336 |
100 |
|||||||
|
360 |
100 |
|||||||
|
384 |
100 |
|||||||
|
408 |
80 |
|||||||
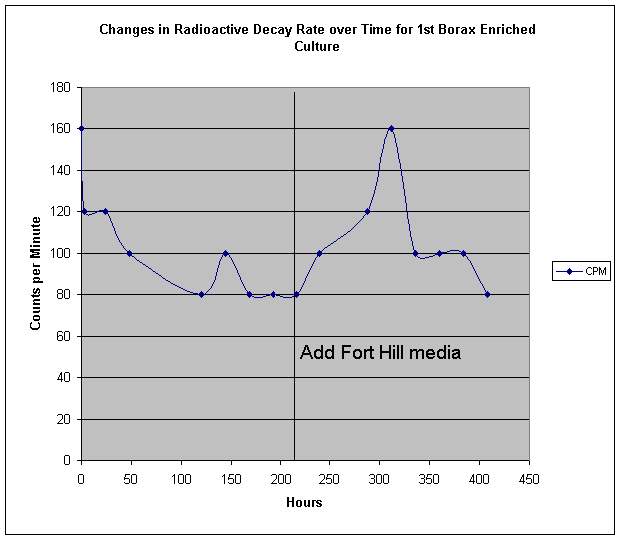
The follow up experiment using Fort Hill fungal stock only did not seem to produce a noticeable temperature drop, however we not that the life cycle and speed of the Fort Hill ascomycete is much slower than the highly accelerated yeast lifetime. We do however, see a moderate change in radioactive decay rate:
|
2nd Boron Enriched Experiment - Fort Hill fungus only |
|||||||
|
100 ml soy milk in shallow plastic tub |
|||||||
|
20 ml half saturated thorium nitrate:H2O |
|||||||
|
1 tsp. sugar |
|||||||
|
2 grams of commercial borax |
|||||||
|
1 ml of crushed Fort Hill medium in soy milk |
|||||||
|
Date |
Hours |
CPM |
Comments |
||||
|
16-Jun |
0 |
280 |
start |
||||
|
24 |
320 |
cheesy |
|||||
|
48 |
400 |
||||||
|
72 |
460 |
||||||
|
120 |
440 |
||||||
|
168 |
440 |
Grey green mat forming |
|||||
|
192 |
440 |
||||||
|
216 |
460 |
||||||
|
264 |
440 |
||||||
|
288 |
400 |
||||||
|
336 |
380 |
||||||
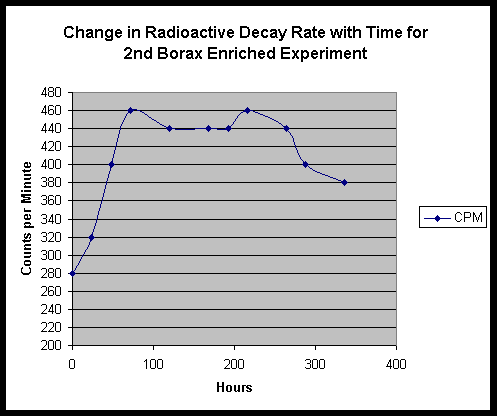
EDS Results:
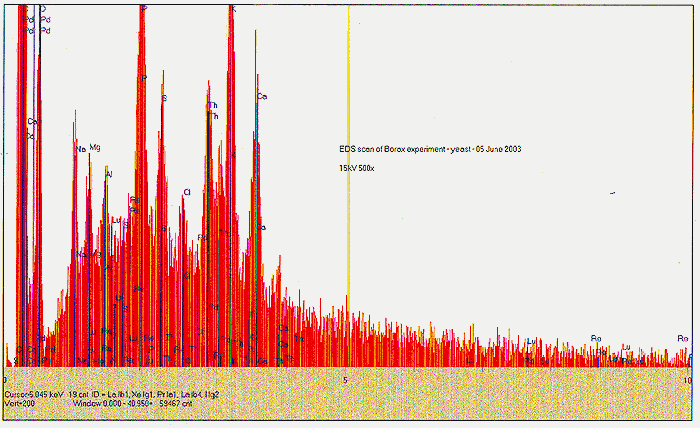
EDS was performed on residue from the first borax experiment, following the period of yeast growth. We find small traces of Pd and Lu, and a stronger signal for Re. EDS performed on the shallow tub Fort Hill - only culture material shows a different set of traces - Bi and a possible very minor peak for Os.
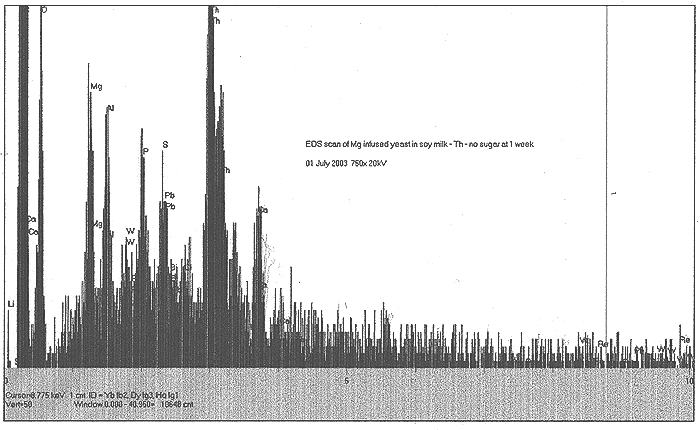
The Role of Magnesium:
It has been hypothesized that particular elements present in biological systems are utilized to facilitate low energy transmutation. One excellent reference came our way in June, and is posted by Rex Research: http://www.rexresearch.com/goldfein/goldfein.htm
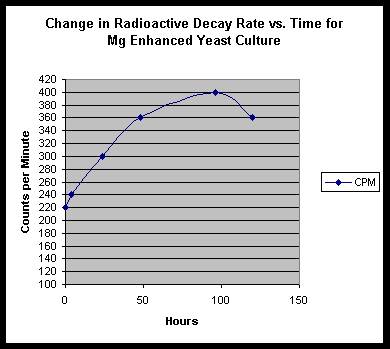
In this work by S. Goldfein, Mg is cited as an important component in biological transmutation. We decided to examine the role of Mg in our experiments. A "standard" culture of soymilk, thorium nitrate and yeast was started and monitored, with three grams of metallic magnesium shavings lying in the beaker bottom. Results are shown thus:
|
Magnesium Enhanced Culture |
||||||
|
100 ml soy milk in beaker |
||||||
|
20 ml half saturated solution thorium nitrate in H2O |
||||||
|
1 packet of baker's yeast |
||||||
|
NO extra sugar added |
||||||
|
3 grams of Mg shavings added to bottom of beaker |
||||||
|
Date |
Hours |
Max CPM |
Comments |
|||
|
26-Jun |
0 |
220 |
start |
|||
|
26-Jun |
4 |
240 |
moderate foaming |
|||
|
24 |
300 |
|||||
|
48 |
360 |
Mg shavings displaced |
||||
|
96 |
400 |
to top, in foam. |
||||
|
120 |
360 |
|||||
EDS Results:
We do find a strongly enhanced signal for Mg, indicating that we were indeed infusing Mg salts into the culture. However, in addition to this, we find slight traces of Pb, Bi, W, and very speculatively, Re.
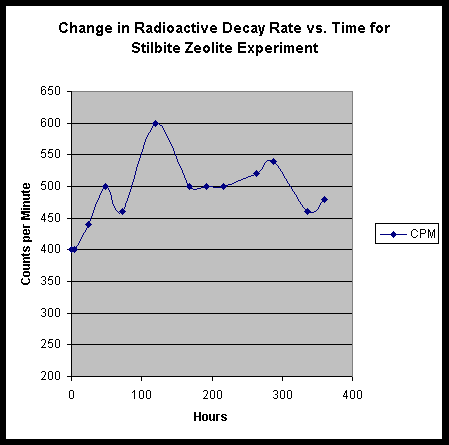
Examination of the Effects of Zeolite Minerals:
The interesting influence of TiO2 nano-particle media prompted us to consider the possible roles that micro-structured inert additives might play in an activated culture. With the TiO2 nano-particles, we were adding inert particles that represented structures in the size range of the mycelia itself. If we were somehow invoking vacuum forces in the alteration of the radioisotopes, further nano-scale particles might augment the process. We then wondered about the inverse! Would the addition of solid inert material containing nano-cavities - and thus fantastically high potential surface area - provide some interesting augmentation? We decided that such a material would best be represented by a member of the zeolite family.
We blended up a standard culture, using soy milk, sugar, thorium nitrate, and yeast; and added to it about 5 grams of broken up stilbite zeolite crystal. A mistake in preparation, however, may have sent the experiment into an awry state. The solution of thorium nitrate in H2O that was used was not the usual half saturated, but instead was a fully saturated solution. Thus, our starting medium was quite radioactive by comparison. Nevertheless, the following table shows our results:
|
Zeolite Experiment |
|||||||
|
100 ml soy milk in beaker |
|||||||
|
1 tbsp sugar |
|||||||
|
25 ml of thorium nitrate:H2O (ended up being fully saturated, not diluted by half) |
|||||||
|
1 pack of baker's yeast |
|||||||
|
5 grams of broken up stilbite crystal, added to beaker |
|||||||
|
Date |
Hours |
Max CPM |
Comments |
||||
|
16-Jun |
0 |
400 |
|||||
|
16-Jun |
4 |
400 |
Thick, but not very foamy |
||||
|
17-Jun |
24 |
440 |
|||||
|
48 |
500 |
||||||
|
72 |
460 |
||||||
|
120 |
600 |
Inversion of separated layers |
|||||
|
168 |
500 |
liquid moves to top |
|||||
|
192 |
500 |
||||||
|
216 |
500 |
||||||
|
264 |
520 |
||||||
|
288 |
540 |
||||||
|
336 |
460 |
||||||
|
360 |
480 |
||||||
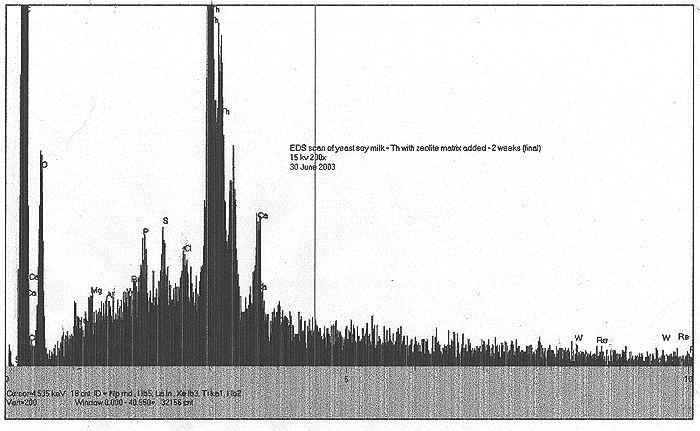
EDS Results:
We find very minor peaks for W and Re.
Two Experiments Showing Negative Results:
In the early spring of 2003, it was suggested by a correspondent that the suspected role of potassium in biological transmutation should be considered in light of our results with Li. Thus, we prepared a culture of yeast in soy milk / sugar / Th / using KCl in lieu of LiCl as an additive. We were surprised to see that very little variation occurred in the radioactive count rate (less than 5% deviation from starting value) and no visible signatures of interesting elemental products were found by EDS. In the case of our yeast process, does added potassium suppress the overall effect?
We also spent considerable time and energy attempting to run a basic yeast / soymilk / sugar experiment with a small amount of americium (from a smoke alarm element) instead of Th or U compounds. The primary difficulty in this procedure ended up being an inability to dissolve the Am from the smoke detector emitter into any acidic or alkali solution at our disposal. We finally prepared a culture and added a few small crumbs (perhaps a couple milligrams) of the Am in its solid form to the medium. Radioactive CPM could not be ascertained, as the soymilk culture appeared to effectively block the decay products of the Am from leaving the beaker. No interesting elemental signatures were found by EDS.
Scaling Up - an Attempt to Produce a Measurable Amount of Transmutative Product:
By late June, we realized that our best chance at conclusively proving the presence of transmutative elemental products was to conduct a volumetrically scaled up experiment. More specifically, after appropriate chemical reduction or processing, we would hope to obtain a much more easily measurable amount of some very surprising element, such as one of the platinum metals we had seen hints of. Ideally, our ultimate goal would be to produce a physical ingot or button of one of these materials. We focused on several metals that had appeared frequently since the earliest runs in this project: rhenium, rhodium, or ruthenium.
By early July, we had entered a final down-select phase of the overall project, and made a final decision on the recipe we chose to attempt in a scaled up version. We elected to scale up our basic boron enriched recipe, using commercial borax, yeast, soymilk, sugar, and thorium nitrate. It was this recipe that appeared to give us the most hope for a sizeable quantity of Pt group product.
On July 3rd, a solution was prepared in a three-gallon plastic bucket, and a yeast culture was initiated. Our recipe:
Two gallons of plain soy milk
500 ml of granulated sugar
250 ml of commercial Borax
500 ml of one half saturated thorium nitrate: DI H2O
Six packs of dried baker's yeast.
The blend was stirred and within a few minutes, became soupy, to the consistency of perhaps a thin milkshake. Slow bubbling and foaming began to show within 2 hours. Our Geiger counter was used to make a quick initial check of CPM at one inch height over the contents' surface, which was found to be about 200 - 220 CPM.
The bucket was transferred to NR's home location by late afternoon. We discovered that the CPM had begun to go into its "decline" mode, and had dropped to 160 to 180 CPM. It had not changed appreciably by the next morning. The bucket was covered loosely with a cardboard sheet, and was locked in a workroom for the next 10 days, whilst NR went on vacation.
Upon NR's return, it was found that the solids of the culture had separated and constituted a bready, somewhat granular soft mass that was resting on a substantial amount of clear yellowish liquid. All foaming or bubbling had ceased. We took EDS spectra for small dried portions of the bready mass as well as the liquid component. Results are shown below:
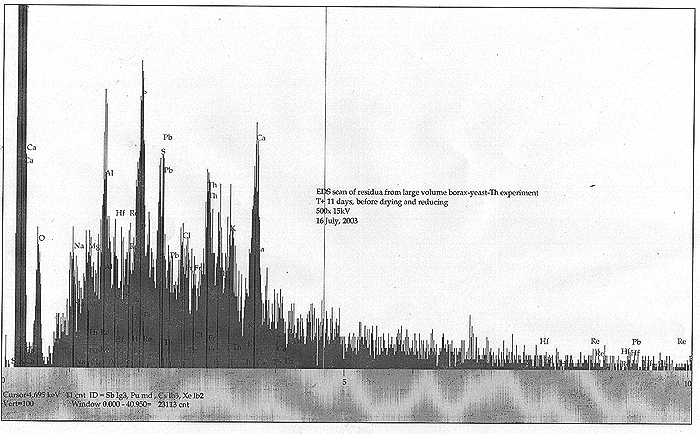
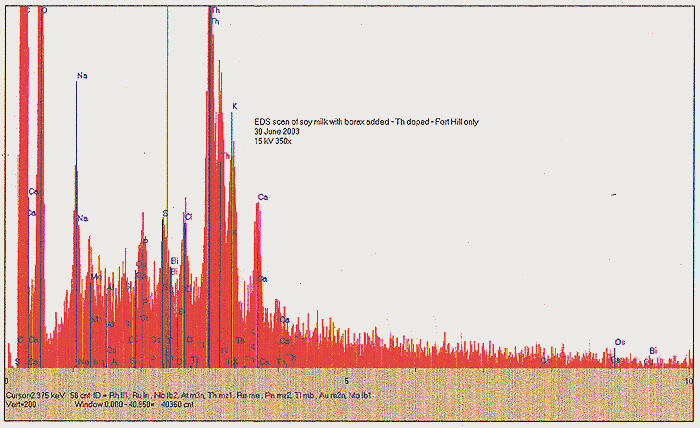
We find some very interesting spectra! Both solids and dried liquid were somewhat similar by EDS, although the dried liquid lacked most of the C, Al, and Ca. We find an apparently significant peak for one of our "target" metals, Re. Also seen are indications of Hf, Pb, and a minor peak corresponding to the typically very short lived Fr. Was our culture still "working", and Fr a transition product? We must note that Fr was indeed observed before, back in our TiO2 nano-particle infused culture (Fort Hill fungus phase).
The contents of the bucket were poured into a large shallow plastic trough and allowed to evaporate with help from a heat lamp, sunlight, and a fan. When the liquid had evaporated, and the solids had achieved the consistency of moist bread pudding, we transferred the mass to a pyrex dish, and continued to dry it out over several more days on a hot plate. We finally achieved a mostly-dried crumbly mass looking like dried browned hamburger, about 1 liter worth. An EDS scan was again taken at this point, and we discover that the Re signal is still strong, however, we now find that the Hf and Fr have disappeared, but a slight peak for Rh may be seen.
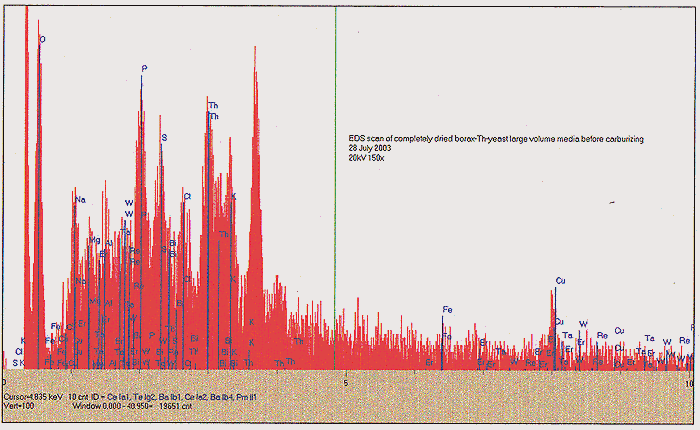
About one half of the dried but unbaked material was removed to an open-ended ampoule, and baked in a tube oven at 500C for 2 hours, with a flow of N2 present. We again find a substantial mass loss, in the form of syrupy and foul smelling fractions that condensed at the cold end of the tube oven. Following this step, we finally obtain about 120 ml of carbonized media, ending up looking like fish tank filter charcoal. SEE PHOTO C. The carbonized media was tested again with our Geiger counter, and found to be significantly "hotter" - about 1400 CPM at one-inch distance. We presume that this enhancement may be due to further concentration of remaining Th. Yet another EDS scan shows us something very intriguing, however; after the 500C annealing, we have lost all noticeable signal for Re and Rh. A slight amount of K and Ca may yet be seen, as well as Th, however we find a very significant amount of Al!
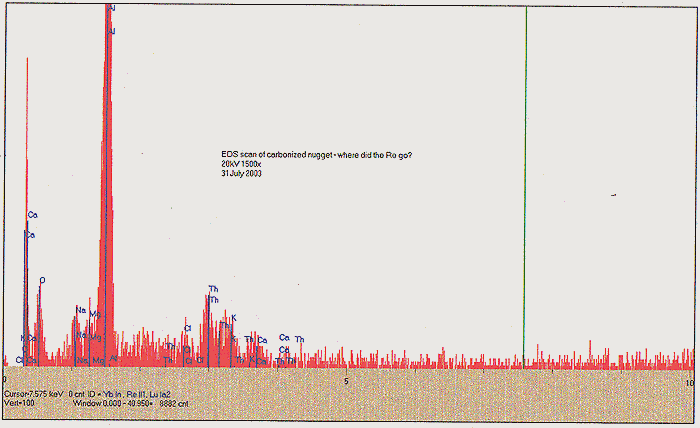
At this time, we are attempting to understand the significance of this change, before proceeding further with processing of the remaining dried media. After all, our primary objective in this experiment was to refine or demonstrate a significant amount of platinum group metal from media at hand. Did we somehow lose Re and Rh into the volatile fractions? This does not seem intuitive. SPF and others have proposed that we may be invoking exotic spin states for elemental materials, and that by intense heating, high spin state materials may be either be yielding up lower energy elemental products, or collapsing into a lower spin state. It was recently suggested also by one of our regular correspondents that further processing of dried Re containing media should be carried out in an oxidizing atmosphere, and that it would be best to convert any platinum metals first into their respective oxides before separation techniques would be employed.
Because of changes in circumstance, at the time of this writing, further EDS work will be highly sporadic, although it remains available for key analysis. We have decided that the time may be opportune for our results to be tendered as widely as possible into the public domain, in the hope that other individual researchers, labs or departments will find interest in "picking up the torch" so to speak. Our immediate plan is to continue processing the Re containing media by following an oxidizing route. Further results will be openly reported.
General Conclusions and Discussion:
This document is intended to provide a "wrap up" or logical capping of our results in this arena, since 2002. Circumstances of employment and lab availability have changed recently, however not before we were able to finish such EDS analysis as we found valuable. If we examine the totality of our work, as reported in our four previous papers on the topic, as well as the technical contents of this document, we find ourselves capable of making a concise set of statements and assertions:
We have continued to work on theoretical modeling of this phenomenon, however a variety of potential mechanisms still lie before us. We have considered the role of the size scale of ascomycete fungal forms, with cellular components reaching down into sub-micron size ranges where vacuum forces and quantum uncertainty may begin to manifest under special circumstances. We have considered the role of additives such as Li and Mg that may enhance the effect by providing proper chemical energy to fungi. We have considered the possibility of some relationship to claims of specific biological energy, as well as altered spin states for atomic species. In particular, the disappearance of certain trace platinum group metal spectra from EDS with heating or carbonizing of fungal media is suggestive of some of the claims by Hudson, et al, regarding the behavior of modified spin state or ORME materials. A careful evaluation of this work is in progress by us. We continue to speculate.
Ultimately, we have presented what we believe to be a set of information and claims that should be easily within the reach of even "home-based" researchers, working in their own shops or kitchens. From grass roots levels up to fully equipped biological laboratories, the possibility of replication and further breakthroughs is present. As always, we give foremost consideration to the potential for new and helpful technology and industrial processes. From nuclear waste remediation, to the production of rare platinum group or heavy metals, the spin-offs from an optimized biological transmutation process are difficult to fathom in their breadth and value!
In closing, we would like to acknowledge and thank the following correspondents, supporters, and intellectual contributors: Dr. Harold McMaster, Dr. Hal Fox, Mr. Jones Beene, Mr. Colin Quinney, the members of Bill Beaty's Vortex Group, Dr. Edmund Storms, Mr. Jeffrey Putz (for SEM and EDS help), Ms. Lori Ann Lothian, and Ms. Lisa Hutchins.
Resources:
Energy Development From Elemental Transmutations In Biological Systems by Solomon Goldfein, U.S. Army Mobility Equipment Research & Development Command, Ft. Belvoir, VA Report 2247 (May 1978)
Transmutation of Radioactive Waste by Louis Kervran, Biological Transmutations, date unknown, published on-line by Global Deactivation of Radiation: http://www.gdr.org/deactivationofradiation.htm
Errata and Addendum for Fifth Report:
23 August 2003
N.Reiter
In our document, we reported on the appearance of Re in several instances, and referred to rhenium as being a member of the platinum metals. We would point out that while occupying a place on the standard Periodic Table near the platinum metals, it is not technically considered to be a member of this group.
We would also like to add one further speculation to our on-going attempt at modeling our observations. Dr. Faile points out that while an altered spin state or orbital structure mechanism might account for the disappearance of Re and an enhancement of Al in our large volume boron enhanced experiment, another general possibility may exist. Exotic stasis fields at an atomic level may be developed during biological transmutation, and that abrupt heating of the organic media and transmuted species might provide enough energy to break this stasis. Once broken, a rapid collapse into lighter more stable nuclei might occur.
