Click here for Biological Transmutation
Click Biologische Transmutation : Biologische Transmutation (auch Kervran-Effekt) werden Kernumwandlungen (Transmutation) in biologischen Systemen (Pfanzen, Mensch oder Tiere).
_____________________________________________________________________________________
Current ver. 2.00, 28/02/2004, ver 2.01, ver 2.02 11/05/2004, ver 2.03 07/01/2007, ver 2.03 18/01/2007, ver 3.00 with an Indroductory Summary, below'
THE MOST BEAUTIFUL THEORY OF PHYSIOLOGY THAT MEDICAL UNIVERSITIES ADOPT IN THEIR TEACHING PROGRAMS
Introductory
Summary
The chemical action of oxygen is called oxidation. However,
oxidation is known to be toxic. This is the reason antioxidants are
so popularly taken to cancel oxidation. This raises a fundamental
question: Is oxygen for life or is it against life ?
This question is also supported by the fact that in certain cases
oxygen is indeed against life. For example oxygen released by Oxygen
Hyperoxide H2O2 is disinfectant, killing
various micro-organisms.
So what is the answer to this puzzling question ?
The answer is: There two roles of oxygen one bad and one good. The bad role of oxygen is indeed its chemical action called oxidation. The good role is an nuclear action of oxygen, not so much known to the scientific community, which we call Oximutation and describe bellow.
THE
SODIUM POTASSIUM TRANSMUTATION,
IN PLACE OF THE SELF CONTRADICTORY CONCEPT OF SODIUM POTASSIUM PUMP
OXIMUTATION
(in Greek the oxygonopyrinosis of
natrium to kalium)
THE NATRIUM POTASIUM OXYGEN-PYRINOSIS
THE GOOD ROLE OF OXYGEN
For this presentation the author was officially nominee for the Right
Livelihood Award (The Alternative Medicine Nobel Prize) in the year 2005.
.
The dual role of
oxygen Oximutation - Oxidation is added, after the 6 hour lecture of
the author at the Zurich PAPIMI
International Conference of 28/2/2004, of the author, see below)
(Natural distribution of K isotopes is
added. Comments about Pollution and Toxicity of Chemical Oxidation are
added. Minor references - Animals that practically take no Oxygen
(click towards the end), Notice for formula's
E=mc2
correctness, click TOP of
HOME PAGE.
This Page is an HTM fast version from the Author's Breakthrough Power Point Presentation
at the International Conference "On PAPIMI Therapies",Salzburg,
Austria, 1 - 3
/11/2002.
This page is a Step in and Stand Up Voice by Acad. Prof. Prof. Dr. Panos Pappas for Scientific Truth against Contradiction and Tolerance in Scientific Knowledge and Critical Sciences.
This theory is also published in
U.S.A. patent number :
7,151,372 B2
YOUR SUGGESTIONS
ARE VERY WELCOME HERE
info@papimi.gr
isnot functional
![]()
Criticism against
(none yet, several years later) or Supporting evidence
(a lot received today) is very much invited.
Submit your criticism or support with or without the indication to be published
here, to:
![]() ,
,
![]() info@papimi.gr
isnot functional
info@papimi.gr
isnot functional
THE MOST BEUTIFUL
THORY OF PHYSIOLOGY THAT MEDICAL UNIVERSITIES ADOPT IN THEIR
TEACHING BODY
Oximutation and Oxidation
The dual role of
Oxygen
in Biology
by Acad. Prof. Prof. Dr. Panos Pappas
Current ver. 2.00, 28/02/2004, upgrated after the 6 hour
presentation lecture of the author at the Zurich International
Conference on PAPIMI on 28/02/2004, at the Technopark, Technoparkstr. 1, CH
8005, Zurich.

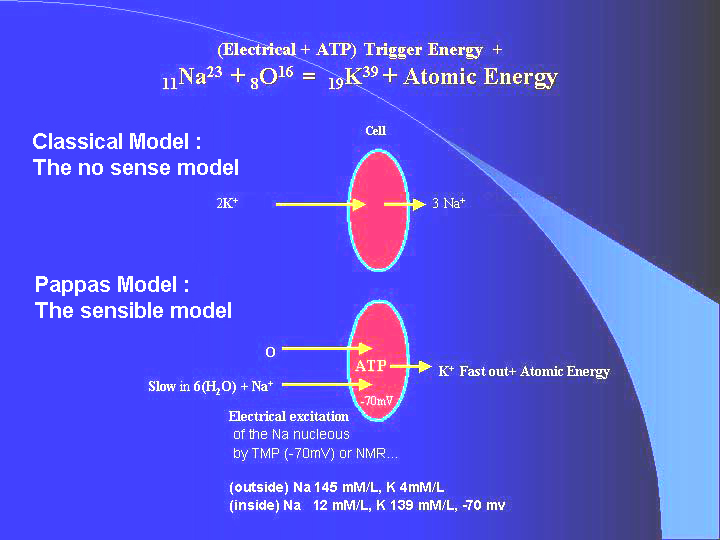
Summary:
The Cell is an TMP (Trans Membrane Potential) controlled microscopic Nuclear Reactor, turning atomic energy directly into electricity TMP back. Our proposal is the very first mechanism we know worldwide for a nuclear reactor to turn atomic energy directly into electricity. The nuclear fuels are O and Na entering a nuclear trans-mutation inside the cell, which we first coined into the term oximutation. The nuclear waste is K. The ignitors are, electromagnetically: TMP, and/or external EM fields like those of PAPIMI, chemically: ATP, Glucose, (controlled by a chain factors: insulin, adrenalin, relevant hormones). This model surpasses the classical ad-hoc model of sodium-potassium pump for the cell in too many and too overwhelming number of paradoxes of the classical model, which becomes non comparable to the present model of oximutation.
The present model not only is free of the "too many paradoxes" of the
classical model, but, also connects logically and explains naturally
many so far apparent unconnected facts. For example, why hyperkalemia
causes heart arrest and death, why hypernatria increases energy and
blood pressure, why cancer relates to hypernatria and free radicals,
why we consume so little oxygen and we do not pollute by breathing
compared to a oxygen+fuel engine, why we spent much more energy than
the intaking energy (chemically) from food, why fishes do not release
CO2 bubbles, why we release kallium while taking only
saline water, oxygen and glucose (a person in a coma), why kalium
kills and natrium energizes, why oxygen is anticeptic and kills
microrganisms, why we should take relatively small quantitities (mgrs
per day) of antioxidants against oxygen's massive (Kgrs per day)
oxidation in our body.
Fact 1: Penetration mobility of Na+ via cell membrane is much less than penetration mobility of K+, because Na+ , holding six molecules of water becomes, so to speak, a "very big and slow molecule" compared to K+.
Fact 2: This is, also, true for the
mobility of both Na+ and K+ escaping through kidneys.
So, practically, concentrations of Na+ and K+
outside the cells is controlled by kidneys, under normal conditions of
kidneys.
Na+ concentration is high, K+ concentration is
low, outside the cells.
Fact 3: Concentration of Na+
inside the cell is controlled by the Na+ mobility (low)
going in and out of the cell by osmosis, assisted by the acceleration towards
the cell by the negative electrical field of the cell TMP, normally,
-70mV; and minus the rate of Na+ transmutating in K+.
Thus, concentration of Na+ is low inside the cell, provided
transmutation rate of Na to K is adequate.
Fact 4: Concentration of K+
is controlled by the transmutation of Na+ to K+
,
minus the mobility K+ (high) escaping to the outside the
cell, delayed by the de-accelaration of escaping K+ by the
negative potential TMP of the cell.
Kinetic energy of positive K+
is lost, leaving the -TMP field, while -TMP is increased by the
escaping positive K+ ,
(because they leave net negative charge behind)
Kinetic energy of positive Na+
is increased entering the cell by -TMP, while -TMP is decreased,
(because while attracted and accelerated, they decrease the net negative
charge inside the cell).
Thus, the excess (after the exothermic nuclear reaction) energy of escaping K+ is turned into a net electrical energy in the form of -TMP.
The cell is a (spherical type) capacitor.
TMP is its charged voltage, due to its net charge inside it.
Considering Na+ , K+
as unit (one electron) charge carriers, and
neglecting minor other ion charge transporters,
We have the obvious condition of steady state equilibrium for the cell's TMP:
-TMP increases, and reaches a steady value, when the slow inwards rate
of Na+ reaches the high
outwards rate of K+, (which,
again K+ , is actually a Na+
transmutation by O to K+ -
Oximutation) , under the above conditions and facts !
Steady state of
TMP
Rate in of Na+ = Rate out of
K+
= Rate of oximutation of Na+
to K+
continuous...
For some relevant details see also the articles in this
site:
"THE EQUATION OF LIFE PART
I"
"THE EQUATION OF LIFE PART
II"
Please, note: At the time the above articles were first written,
Louis Kervran critical error was not realized yet, see also below.
Major Contradictions for the
Classical Model:
Related to Human and Animal
Energy
***
Important Note to avoid confusion:
Usually in textbooks of nutrition and in related literature, the following confusing notation is
used:
1Cal= 1cal=1kcal=4,2 KJoules !
instead of the correct:
1cal=4.2Joules=1 celsius degree rise/gr of H2O
from standard physics definition.
Also in references of nutrition, "1calory" or "1 cal" or "1 c" may mean
(usually without warning):"1000 calories" or "1000 cal" = 4,2 KJoules !
We apologize for the confusion that is arising, but, it is not our preferred or intended notation
!
***
Estimated Energy needed for the human heart
and vein-artery movement for blood circulation:
70 wattsx3600x24 secs = 70 watts x 86400 secs
6.048 KJ = 1.440 Kcal a day = 1.440 Cal a day
Energy needed for Brain:
25watts x 86400 = 2.160 KJ = 514 Cal a day
Energy for Breathing and Body Heating:
another 500 watts to 1000 watts
corresponding to 43200 to 86400 KJ/Day
10.282 to 20.572 Cal/Day.
Important Notice: The human body has normally to be in an environment
of 25 to 26 degrees Celsius, about 11 degrees less than its normal
temperature - 36.6 degrees, to feel comfortable, thus to be constantly
able to keep a rate of losing heat (dissipation of heat) to a cooler environment of
about 11 degrees.
Minimum Needed Energy Estimation just for living:
12.500 Cal/Day without any work.
The above figure is only an
approximation of ours. However, it proves our point, even it is an
overestimation and actually it is less than half of the above proposed
value.
Recent data, based on olympic athletes, exercizing daily for the
August 2004 Olympics, indicates:
Daily work spending >1000000 Cal
Daily controlled and strict intaking food calories <2000 Cal !
Contradiction:
Actual average nutrition intake:
600 to 2.000 Cal/Day !
The above contraction is excused in the literature by claiming the Human
and Animal Organism is
the highest efficient energy engine.
What a myth!
The Human and Animal Organism is the most Energy Wasting System,
having and using huge atomic energy resources.
Click here
for a Detail Analysis - New
*****************
Other serious contradictions
OOOOO
EXACT ATOMIC MASSES
for the related isotopes of Na, O, K
("HANDBOOK of CHEMISTRY and PHYSICS" 82-nd Edition © 2001 by CRC Press
LLC, Section 11, page-52, 59.)
and calculation of Atomic Energy by
formula:
E=mc2
Calculations confirmed by Gregory Hatzis, Physicist.
Notice: Results are correct to
the same degree as the above formula is correct (?)
click TOP OF HOME PAGE.
Na23 = 22,989769700000
100% (only one
isotope)
O16 = 15,99491462200 (99,757%), Leading to
K39 =
38,963706900000 - natural abundance: 93,2581%
O17 =16,999131500000
(0,038%),Leading to
K40 =
39,963998700000 - natural abundance: 0,0117%
O18 =17,999160000000
(0,205%), Leading to
K41 = 40,961826000000 - natural abundance:
6,7302%
Mean mass for O = 15,999404927439,
K mean value from above =38,9637069x99,957+ 39,9639987x0,038+40,961826x0,205
=38,968182
K books' mean value = 39,098300000000
MASS CHANGED INTO ENERGY
For O16: DM = 22,9897697 + 15,994914622 - 38,9637069 =
0,000020977422 Kgr/Mol (SI Units)
99,757%
For O17: DM = 22,9897697 + 16,9991315 - 38,969987=
0,00002490125 Kgr/Mol (SI Units)
0,038%
For O18: DM = 22,9897697 + 17,99916 - 38,9681823=
0,0000271037 Kgr/Mol (SI Units)
0,205%
which, using E=DMC2
, C=299792458 m/s for the velocity of light, leads to exothermic (giving out energy) reactions for Na and all Isotopes of
O, as follows:
PAPPAS' EXOTHERMIC NUCLEAR REACTIONS:
11Na23 +
8O16 =
19K39 + 452.484
Kcal/mMol 99,757%
11Na23 +
8O17
= 19K40 + 537.149
Kcal/mMol 0,038%
11Na23 +
8O18 =
19K41 + 584.629
Kcal/mMol 0,205%
Mean: 11Na +
8O =
19K
+ 452.787 Kcal/mMol
***************
Louis Kervran's Critical Error
Using the mean book values for O and K (=39,0983)
Dm=0,109130000000 gr/Mol
which leads to an endothermic (absorbing energy) reaction for Na and
O.
Obviously this is a wrong result, because the mean book value for K=39,0983
("HANDBOOK of CHEMISTRY and PHYSICS" 82-nd Edition © 2001 by CRC Press
LLC, Section 11, page-52, 59.)
is not the mean value for K=38,9637069 given
above, assumed to be produced in the
body, assuming all naturally occurring isotopes of oxygen give nuclear reactions with equal probability in the
body. Obviously, in such a case of equal probability, the frequencies of K isotopes
assumed to be produced in the body are equal to the corresponding frequencies of
O
isotopes.
Therefore, the mean value of 38,9637069 given above for K isotopes,
assumed to be produced in the body is not the book given value
K=39,0983 of nature.
Louis Kervran, apparently using the book mean value for K, was led to the ironic
and wrong result that the nuclear equation which he first
proposed, is an
endothermic one.
Click Here: For
LOUIS
C. KERVRAN
HISATOKI KOMAKI
"The above correction from an endothermic to an exothermic (producing energy out)
nuclear reaction for Na and O seems to be the missing link to a complete understanding of the physiology of the cell and its atomic
energy"
Click Here for Frequently
asked Question
Obviously, even if, the occurring probability for all oxygen isotopes to enter to one Pappas Nuclear reaction is not equal as we assumed
above, the particular occurring reaction is
always exothermic releasing the same order of energy as the
assumed mean value for energy
of:
452.787 Kcal/mMol
Therefore, for any other (different) relative percentage for the Pappas' Nuclear equations, actually occurring in the body will lead to slightly different, but not significantly different, results than the above figure, which may not change the status of the present theory.
*************
Important Test and Confirmation of the present Theory:
Any deviation of
isotopic natural occurrence of K in the body
- from that provided by nature:
K39:
93.2581%, K40:
0,0117%, K41:
6,7302%
HANDBOOK of CHEMISTRY and PHYSICS" 82-nd Edition © 2001 by CRC Press
LLC, Section 11, page-52, 59
will
prove that K is nuclearly produced in the body and not all received
from nature!
ANSWER
14/2/2003
Indeed
the answer of this test is affirmative to the present
Hypothesis-Theory, for the K occurrence in the body is known to be
variant, believed to depend on the individual's kind of food intake.
The isotopic occurrence of K in the body is indeed variant,
individualized and different from the Natural occurrence of K in
Nature:
K39:
93,2581%, K40:
0,0117%, K41:
6,7302%
HANDBOOK of CHEMISTRY and PHYSICS" 82-nd Edition © 2001 by CRC
Press LLC, Section 11, page-52, 59
This proves K isotopes are produced in animals and not all K
isotopes are received from nature. The occurrence of K produced will
be that or closed to that of the corresponding oxygen occurrence:
O16:
99,757%, O17:
0,038%, O18
: 0,205%
This makes their occurrence
obviously different than the nature's occurrence. Animals and
vegetables - (with no known oxygen intake, therefore, no Na+O to K) -
serving as food to men will offer a different isotopic composition,
depending on the individual's food preference, animal or vegetable.
This K isotope composition will further individualize, depending on
the individual rate of metabolism-transmutation of
Na and O isotopes to K isotopes.
References: "Isotope Geology", Kalervo Rankama, Pergamon Press, p
302-311 .....
What is presented above strongly presents evidence to support the present theory.
**************
MEAN ATOMIC ENERGY RELEASED PER MASS
E=DMxC2 = 1.885.352 KJ/mMol = 452.484
Cal/mMol of Nacl or mMol of
O2 ,
Cal = Kcal, 1 mMol NaCl = 23 + 35= 58 mgr, 1mMol O2 =32mgr
A.E. = 452.787/58 Cal/mgr Na(Cl) =
7.806 Cal per mgr Na(Cl)
SEVEN THOUSAND EIGHT HUNDRED AND SIX CALORIES
per mgr Na(Cl)
OR IN TERMS OF OXYGEN
A.E. = 452.787 Cal per 0.0224 Liters O2
(per 22mL of O2 )
452.787 Cal/mMol O2 /32 =
14.149 Cal per mgr O2
FOURTEEN THOUSAND AND ONE HUNDRED AND FOURTY NINE CALORIES
per mgr O2
Chemical Energy Released
for Carbohydrates, Proteins, Fats
0,0042 to 0,00945 Cal per mgr
or
4.200 TO 9.450 Cal per Kgr
Recommended about 2000 Chemical Cal per day.
****************
The dual role of oxygen
Therefore, oxygen may enter two types of reactions:
1. Oxidation:
This a
Chemical reaction - Oxygen
burns
Carbohydrates, Proteins, Fats
producing H2O, water and CO2, carbon dioxide a
gas, (however, not seeing much for fishes..., no CO2 bubles
seeing for the gold fish...)
2.
Oximutation:
This a coined term by us from oxygen's
nuclear trasmutaion
into K, according to O + Na =K.
****
Generally
Chemical burning with Oxygen -
Oxidation - in nature
is very much Polluting. It is also known in certain cases to be
damaging, creating toxic substances (for example: free radicals in the
body) for which Anti-Oxidants
are recommended for their elimination.
Nuclear "burning" with Oxygen
- Oxygen fusion -
Oximutation
involving only tiny quantities anyway, is practically quantitatively sufficient and non Polluting.
In general, the quantity oxygen needed
for the Nuclear fusion of oxygen - Oximutation - to produce the same
energetic result is in the order of 1.00.000 times
less than the Oxygen's Chemical reaction - oxidation.
In other words, the efficiency of the Nuclear fusion of oxygen-Oximutation is in the
order of 1.00.000 times higher than the corresponding Chemical
reaction - Oxidation.
This might explain why so little oxygen for animals and humans is
enough to consume, in order to live and to be active, when Oxygen is
used for Oximutation.
Particularly for fishes, even as big and powerful as the shark, (click
here for "New Scientist Puzzling Article", - sharks and
annimals that practically take no oxygen !!! ,
New Scientist,
vol 177,
issue 2385 - 08 March 2003, page 46)
dissolved Oxygen in the water is enough for their all the time living
and acting under water (mostly + NaCl), with seriously limited
possibility of oxygen supply, a fact that can not be disputed.
****
ESTIMATION OF ENERGIES IN THE BODY
ATOMIC ENERGY IN THE BODY
Oximutation
Higher than
90 TO 95%
CHEMICAL ENERGY IN THE BODY
Oxidation
Less than
5% TO 10%
Ratio of C.E. over A.E. (Nacl)
for the same mass quantity in the range of
1 / 1.000.000 =
x# of mgr/ x# of kgr = 10-3/103= 1/106

The
picture shows two typical household containers, one for sugar and one
for salt.
Sugar, like hydrocarbons, is a typical source of chemical energy
leading into
Oxidation
Salt is a typical source of sodium Na, source of atomic energy, as
explained above, leading into
Oximutation
A few GRAINS (10) coming out of the salt shaker, containing Na,
are energy equivalent to few 10 GRAMS of sugar - several teaspoons,
Surprisingly, they closely reflect the above ratio of
1/1.000.000 = mgrs / Kgrs
of chemical energy to atomic energy ratio !
Excess intake of Na will result in excess availability of energy in the body system, resulting in excess energy storage in the form of fat tissue build up, increase of heart energy output - "pump output,” resulting in higher blood pressure, etc, as it is commonly found and known.
As with every nuclear reactor - (carrier for controlled nuclear reactions, as opposed to non controlled destructive atom bomb) - the nuclear body should also be limited and controlled.
Please
control your Na intake according to your body’s need as your Doctor
recommends. Remembering that the beat of your heart and your very
existence depends on the nuclear reaction of Natrium - (salt, "the
life of earth", according to the holy scripture texts), as much as
life also depends on the corresponding
Oxygen's nuclear participation!
**********
0. ENERGY MEDICINE
SHOULD BE PROPERLY UNDERSTOOD
particularly the dual role of oxygen leading
A. to oximutation good role
B. to oxidation bad role
1. PAPIMI ENHANCES
BODY'S (A) ENERGY
by enhacing oxygen's oximutation
and limiting oxidation
2. PAPIMI ENHANCES
BODY'S METABOLISM
by enhacing oxygen's oximutation
and limiting oxidation
****************
The Human and Animal Organism is the most Energy Wasting System,
having and using huge atomic energy resources.
Click here
for a Detail Analysis - New
Other serious contradictions
Click Here: For
LOUIS
C. KERVRAN
HISATOKI KOMAKI
Click Here for Frequently asked Question
Click here for the related previous articles
"THE EQUATION OF LIFE PART I"
"THE EQUATION OF LIFE PART II"
THE MOST BEUTIFUL THORY OF PHYSIOLOGY THAT MEDICAL UNIVERSITIES ADOPT NOW IN THEIR TEACHING PROGRAMS