rexresearch.com
Home ~ Alchemy Index
Biological Transmutation
Energy Development From Elemental Transmutations In Biological Systems
by
Solomon Goldfein
U.S. Army Mobility Equipment Research & Development Command, Ft. Belvoir, VA
Report 2247 (May 1978)Abstract ~ The purpose of the study was to determine whether recent disclosures of elemental transmutations occurring in biological entities have revealed new possible sources of energy. The works of Kervran, Komaki, and others were surveyed; and it was concluded that, granted the existence of such transmutations (Na to Mg, K to Ca, and Mn to Fe), then a net surplus of energy was also produced. A proposed mechanism was described in which Mg-Adenosine Triphosphate (MgATP), located in the mitochondrion of the cell, played a double role as an energy producer. In addition to the widely accepted biochemical role of MgATP in which it produces energy as it disintegrated part by part, MgATP can also be considered to be a cyclotron on a molecular scale. The MgATP when placed in layers one atop the other has all the attributes of a cyclotron in accordance with the requirements set forth by E.O. Lawrence, inventor of the cyclotron.
It was concluded that elemental transmutations were indeed occurring in life organisms and were probably accompanied by a net energy gain.
Contents
Preface
I. Introduction
(1) Subject
(2) Background
II. Investigation
(3) Plan
(a) Net Weight Change
(b) Energy Required for Nuclear Reaction
(c) Probable Location of Energy Development
(d) Chemicals Involved
(e) Energy Production
(f) Ions Present in the Mitochondrion
(g) Current Flow
(h) Structure of Mg ATP(4) Proposed Alternate Mechanism for Energy Production
(a) Oscillatory Magnetic Field
(b) Presence of Hydrogen Ions
(c) Auxiliary Control & Propulsion of H+ by Dipoles
(d) Induction of Ring Current on Hydrogen Ion
(f) Circular Helical Path of Hydrogen ionIII. Discussion
(5) GeneralIV. Conclusions
(6) ConclusionsIllustrations:
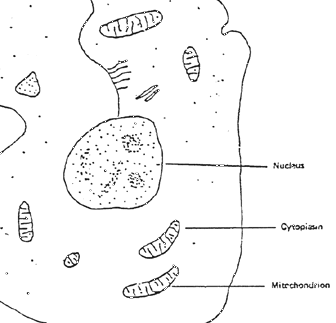
Figure 1 ~ Typical Cell Showing a Number of Mitochondria
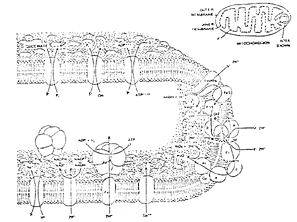
Figure 2 ~ Membrane of the Mitochondria
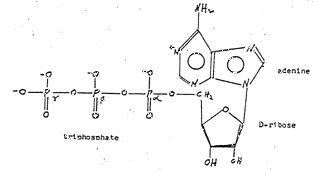
Figure 3 ~ Chemical Structure of Adenosine Triphosphate
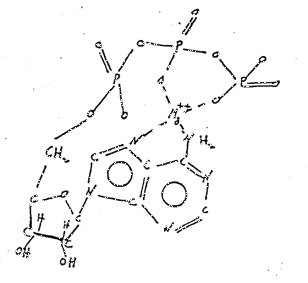
Figure 4 ~ Chemical Structure of Adenosine Triphosphate Chelated with Magnesium Ion (ATP)
Figure 5 ~ Planar View of Two Molecules of MgATP with Six Dipoles
Figure 6 ~ Planar View of Four Molecules of MgATP with Twelve Dipoles
Figure 7 ~ Side View of Four Molecules of MgATP Showing Circular Spiral path of H+
The work described in this report was authorized and funded by the U.S> Army Mobility Equipment Research & Development Command, Material Technology Laboratory, under Project MTL-01901, PMMR-26
Solomon Goldfein was the principal investigator for the effort. The work was under the direction of Emil J. York, Material Technology Laboratory.
Robert C. McMillan, Chief, Radiation Research Group, provided guidance with regard to matters relating to physics and nuclear physics aspects.
(1) Subject ~ The subject of this study was to determine whether recent disclosures of elemental transmutations occurring in biological entities have revealed new possible sources of energy.
(2) Background ~ Two investigators, Kervran and Komaki (1, 2), have recently been nominated for a joint Nobel prize for their work involving the experimental proof that elemental transmutations were occurring in life organisms. Elements which were definitely proven to have been transformed were sodium (to magnesium), potassium (to calcium), and manganese (to iron). Actually, observations have been made for almost 200 years that elemental transmutations were occurring, but little credence was given to them because they resembled alchemy --- a relic of the idle ages.
[(1) Kervran, C.L.: Prev. en Biologie de Transmutation a Faible Energy; Maloin, Paris (1975); (2) Komaki, H.: Rev. de Pathologie Comparee 67: 213 (1967); 69: 29 (1969)]
From Jeuneman (3), investigations on transmutations can be traced from 1799 to the present.
[(3) Jueneman, F.B.: Industrial Research 19 (13): 11 (Dec. 1977), "The Transmutation of Species".]
The French chemist, Vauquelin, in 1799, observed a large quantity of lime in the daily excretion of hens. To determine where the lime was coming from, he fed a captive hen a diet of nothing but oats. He measured the amount of lime in the oats, fed the oats to the hen, and then measured the amount of lime in the excretion and the eggs of the hen. The lime had increased fivefold. Vauquelin realized that lime had been created but he did not know why.
In 1822, Prout, an English experimenter, defined the transmutation of elements by studying incubating chicken eggs. He observed increasing amounts of calcium carbonate inside the egg and determined that the compound did not come from the shells.
In 1831, Choubard experimented with germinating watercress seeds. He observed new minerals in the young plants that were not present in the original seeds.
In 1844, Vogel also worked with watercress seeds. He placed his seeds in a controlled-air environment and added a sulfur-free nutritive solution to the seeds. The plants that grew from the seeds were found to have more sulfur than the original seeds. Vogel's answer was that sulfur was not a simple element or that sulfur was introduced from sources unknown.
After Vogel, Lauwes and Gilbert observed the weight variation of ashes during vegetation. In particular, they found a variation in magnesium.
In 1875, von Herzeele also studied vegetating plants and discovered a weight increase in the ashes of the young plants.
Later, following the works of Vogel and Lauwes and Gilbert, von Herzeele considered the variation in the weight of magnesium. He concluded that a transmutation of elements had taken place.
In 1960, Baranger, of France, published his findings on variations of calcium and phosphorus in germinating seeds. He also concluded that a transmutation had taken place, but he did not determine the reason.
In the early sixties, Louis Kervran's discoveries on transmutations were published by a French scientific review. Kervran demonstrated that not only molecules but also atoms could be transformed, and he verified the transmutation of matter from atom to atom. (4)
[(4) Jueneman, F.B.: Industrial Research 19 (13): 11 (Dec. 1977), "The Transmutation of Species".]
Komaki reported that eight strains of microorganisms grown in K-deficient culture media increase the total K by converting Ca to K --- a fission reaction. A theoretical basis for Kervran's work has been discussed by de Beauregard. (5)
[(5) de Beauregard, O. Costa: Proc. 3rd Intl Cong. Psych. (Tokyo), p. 158 (June 1967)]
Working at the Ecole Polytechnique in Paris, Baranger, who also headed the organic laboratory, determined that transmutations were indeed taking place. Yet, none of these latter-day alchemists could propose a theory for the mechanism of such an unlikely appearing event as biological transmutation.
Summarizing, disclosures have been made of transmutation in microorganisms, plants, and animals. Some have been fusion while others have been fission phenomena.
(3) Plan ~ In the search for clues to the possibilities for energy development, calculations were made to determine whether there was a net weight loss during the transmutation. This was followed by an examination of the most likely place for the event to occur and the presence there of the same elements disclosed to have undergone nuclear changes. A study then flowed to determine of a mechanism was present which might cause such nuclear transmutations in accordance with presently accepted nuclear theory.
(a) Net Weight Change ~ The study was limited to fusion reactions so that the difference in atomic number would be only one. These included Na, K, and Mn:
Sodium to magnesium where: Na + H+ » Mg
Potassium to calcium where: K + H » Ca
Manganese to iron where: Mn + H » Fe
The atomic weight differences were:
23/11 Na + 1/1 H+ » 24/12 Mg
22.989 7707 1.007 825 19 23.997 595 39
23.989 770 7 True Atomic Weight
0.007 825 19 Loss of Mass39/19 K + 1/1 H+ » 40/20 Ca
38.963 710 1 1.007 825 19 39.971 535 29
39.962 558 9 True Atomic Mass
0.008 976 39 Loss of Mass55/25 Mn + 1/1 H+ » 56/26 Fe
54.938 050 30 1.007 825 19 55.945 875 49
55.934 936 3 True Atomic Mass
0.010 939 19 Loss of MassEnergy developed as a result of transmutation:
Conversion factor for atomic mass unit (amu) = 931 MeV / amu
Na » Mg 0.077 825 19 (931) (7.29 MeV)
K » Ca 0.008 976 39 (931) (+8.35 MeV)
Mn » Fe 0.010 030 10 (931) (+10.18 MeV)
(b) Energy Required for Nuclear Reactions ~ No information has been discovered as to the energy required for these reactions to occur and, thus, whether there would be a net gain of energy. As far back as 1932, however, it was known that alpha particles having energies when ejected from radioactive elements of medium life of between 5 and 7 MeV could cause atomic disintegrations. Cockcroft and Walton built a tall, vertical tube capable of evacuation with a filament producing electrons at the top and a target of the element to be bombarded at the bottom. A low pressure of hydrogen was introduced and ionized by collisions with the electrons produced by the filament. The top of the tube could be raised to a high positive potential, up to 700,000 volts, the bottom being at earth. On reaching the bottom of the tube, the protons had a kinetic energy equal in electron-volts to the potential in volts at the top of the tube. Using a lithium target, at 45° angle to the bombarding protons, disintegrations of some of the lithium atoms into pairs of helium nuclei ejected almost in opposite directions were observed with only 120,000 volts. (6)
[(6) Cockcroft, J. & Lewis, B.: Proc. Roy. Soc. A, 136: 619; ibid., 137: 229 (1932); ibid., 154: 246, 26 (1936)]
The reaction is:
H + Li » He
Another such pertinent reaction was:
Fe + H » O + He
(c) Probable Location of Energy Development ~ The mitochondrion, a cylindrically shaped organelle, is universally recognized as the primary site for energy production in all life organisms regardless of whether they are one-celled bacteria, plants, or animals (7). Some cells have as many as 7000 mitochondria (Figure 1). The mitochondrion is divided into components in which various reactions take place (Figure 2). (8)
[(7) Mahler, Henry R. & Condes, Eugene: Biological Chemistry VI, 601, 609, 618 (1966); (8) ibid.]
Figure 1: Typical cell showing a number of mitochondria
Figure 2: Membrane of the mitochondria
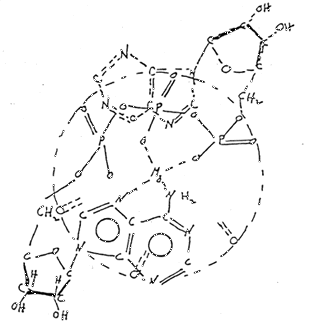
(d) Chemicals Involved ~ Adenosine triphosphate (ATP) is now recognized as the molecule most involved in energy production in this mitochondrion (Figure 3). When complexed with Mg++, it forms a cyclic ATP and proceeds through a series of reactions which produces energy (Figure 4).
Figure 3: Chemical structure of ATP
Figure 4: Chemical structure of ATP chelated with Mg ion
(e) Energy Production ~ The Mg+ is considered to be a catalyst for these reactions. One phosphate group after another splits off so that the resultant chelate becomes a diphosphate (ADP) and then a monophosphate (AMP). The phosphate groups hydrolyze, and the energy of hydrolysis for each reaction is 7500 calories. The D-ribose then splits off and proceeds through a long glycolic decomposition cycle to yield further energy. The chelated, cyclic MgATP thus breaks up completely and is rebuilt through a series of chemical reactions. None of the decomposition and rebuilding cycles is as yet completely understood.
(f) Ions Present in the Mitochondrion ~ Na, Mg, K, Ca, Mn, and Fe ions have been found to be present in the mitochondrion. These are the same ions which have been previously found to undergo nuclear transmutations by Kervran. In addition, another pair, Cu and Zn, differing by one proton (elements numbered 29 and 30) is also present (9). H+ and OH- ions have been produced and are maintained in separate compartments (10). Conversion to Cu and Zn is also accompanied by a loss of mass and thus a production of energy:
6329Cu + 11 H+ » 6430Zn
62.929 592 1.007 825 63.937 417
63.929 145 True Atomic Weight
0.008 272 Loss of Mass6529Cu + 11H+ » 6630Zn
64.927 786 1.007 825 65.935 611
65.926 052 True Atomic Mass
0.009 559 Loss of Mass[(9) ibid.; (10) ibid., p. 609, note 1 ]
(g) Current Flow ~ The net result of the many reactions occurring in the mitochondrion is a flow of electrons (11). This flow is oscillatory in the MgATP crystal.
[(11) Hinkle, Peter C. & McCarty, Richard: Scientific American 104 (March 1978), "How Cells Make ATP".]
Gurney determined that removing a negative ion from the interior of a perfect crystal left a vacant lattice point to which a positive charge is associated. If this point is approached by a free electron in the conduction band, the electron will be attracted as from a positive-charged particle. Thus, if the electron loses energy, it may be trapped in the field of the charge. This trapped electron will undergo a series of stationary states towards a series limit. Beyond this limit, a continuum of states will exist. The periodic field of the lattice will cause periodic fluctuations on the wave functions of the trapped electron, but the trapped electron will not be prevented from having a definite wave function, in each bound state, which will be like that of an electron within an atom in a vacuum and will be spread over many atomic distances in the crystal. (12)
[(12) Gurney, R.W.: Handbook of Physics, ed. Condon & Odishaw, 2nd edition, section 12 (1967), "Ionic Crystals".]
Similarly, Gurney determined that removing a positive ion from within a perfect crystal left a vacant lattice point to which a negative charge is associated. If this point is approached by a free, positive hole in the filled electronic band, the free, positive hole will be attracted. If the free, positive hole loses energy, it will be trapped in the field of the negative charge. A set of stationary states toward a series limit will be encountered by the trapped, positive hole. (13)
[(13) ibid. ]
Thus, in either case the current is oscillatory.
(h) Structure of MgATP ~ The complete conformational structure of mgATP has not yet been elucidated. A key problem has been the function of the Mg+. Kothekar, et al. (14), made a study of the changes in electronic charge and energy distribution in ATP and ADP after incorporation of Mg++. They hypothesized that even though Mg++ is not absolutely essential for enzymatic action, its presence facilitates the action by modifying the charge distribution. The most likely position of Mg++ is symmetrical between the three P atoms. Binding between Mg++ and ATP was considered mostly ionic in nature. No detailed, acceptable information is yet available regarding the crystalline structure of MgATP. (15)
[(14) Kothekar, V., et al.: Indian J. Biochem. & Biophys. 10 (4): 279-282 (1973), "Molecular Orbital Calculatiosn of Mg Complexes with ATP & ADP"; (15) Kennard, O., et al., Proc. Roy. Soc. London A-325: 401-436 (1971), "The Crystal & Molecular Structure of ATP"]
(4) Proposed Alternate Mechanism for Energy Production ~ There is hardly a material in life organisms which performs which performs only a single function. Therefore, MgATP was examined for an additional and alternate means of energy production which would encompass nuclear fusion reactions in accordance with recognized nuclear theory and practice. The principal method of producing nuclear reactions is by an accelerator --- either linear or cyclic. E.O. Lawrence in the 1930s used the cyclotron resonance phenomenon as the basis for the cyclotron particle accelerator. He derived the equations of motion for a charged particle in a uniform, magnetic field with a constant period of revolution so that particles can be accelerated indefinitely in resonance with an oscillatory electric field. Recently, Hunter an McIver developed a small spectroscope based on this principle. (16)
[(16) Hunter, Richard L. & McIver, Robert T.: American Laboratory 9 (11): 13 (1978), "Rapid Scan ion Cyclotron Resonance Spectroscopy" ]
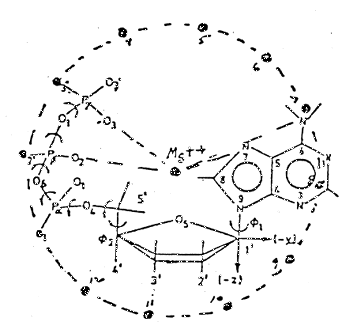
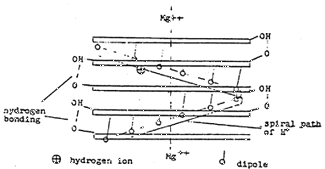
In view of the lack of information regarding the crystalline structure of MgATP or its structure with regard to adjacent molecules of MgATP, it is hypothesized tat the molecules of MgATP are situated one above the other so located that the Mg++ forms a continuous chain. With the exception of the D-ribose unit, the complex or chelate lies in a plane. The D-ribose folds up --- a phenomenon called sugar puckering (17). Since the D-ribose molecules might interfere with each other under these conditions, the MgATP has been rotated so that the D-ribose units would lie 180° apart. A top view of the two molecules is shown in Figure 5. Since the P groups are equidistant from the Mg++, it follows that they may be 60° apart around a circle whose center is the Mg++. The acyl oxygen dipoles attached to the P atoms would likewise be situated around the circumference in which case four molecules of MgATP would be required for twelve dipoles to be present in the circle (18). Figures 6 and 7 show top and side views respectively of such an arrangement. The phosphate chain is in the folded configuration and not extended as in anhydrous, inorganic phosphates. It would form a helix, and torsion angles about the bonds have been calculated (19). The net effect would be that the H+ would make a helical path which would be circular as shown in Figure 7.
Figure 5: Planar view of two molecules of Mg chelated ATP, one directly above the other facing each other. The dashed line structure represents the lower molecule. The Mg++ is at the center of a circle with six dipoles spaced evenly around the circumference --- three per molecule of ATP. The adenine part of the adenosine triphosphate straddles the perimeter
Figure 6: Planar view of four MgATP molecules showing 12 dipoles and only one Mg++ and adenosine molecule. Arrows show alpha, beta, & gamma rotation of P atoms and, hence, dipoles 1, 2, and 3 when not chelated. Chelation prevents rotation of P atoms and could result in forcing dipoles out of the plane
Figure 7: Side view of four molecules of MgATP. Possible hydrogen bonding between OH of D-ribose and gamma oxygen is shown
[(17) Kennard, O., et al.: Proc. Roy. Soc. London A-325: 4010436 (1971), "The Crystal & Molecular Strcuture of ATP"; (18) ibid.; (19) ibid.]
Dipoles have been found to be energized by unsaturation in the ionic structure --- binding with metal ions with each dipole presumable requiring, on the average, one saturation or double bond (20). Hughes and Rideal showed that a dipole is induced in a double bond if it is situated in the alpha position but not if it is further away (21). In contrast, the presence of a double bond in the 4-5 carbon position of sphingomyelin increases the surface dipole and, hence, the surface potential of the monolayer (22). No evidence exists, therefore, for a maximum-distance limitation. In this case, the unsaturation in the adenine molecule comprising four double bonds should, therefore, be sufficient to energize the three dipoles in the phosphate groups. The current formed by the electron flowing in an oscillatory fashion from one Mg++ to another must of necessity be accompanied by an oscillatory magnetic field. This could easily extend out to the perimeter and accelerate the H+ to relativistic speeds. It would be guided in its path by the dipoles.
[(20) Shah, O. Dinesh & Schulman, Jack H.: Advanced Chemical Series 84: 189 (1967); (21) Hughes, A., & Rideal, E.K.: Proc. Roy. Soc. London A-140: 253 (1933); (22) Shah, O.D., & Schulman, J.: Lipids 2: 21 (1967)]
An interesting feature noticed in Figure 5 is the location of the adenine rings in the adenosine heterocycles. They sit directly on the circumference or perimeter of the circle formed by the acyl oxygen dipoles and 180° apart from each other. When a conjugated ring molecule is placed in a magnetic field, its electrons are caused to circulate and, in circulating, they generate secondary magnetic fields, i.e., induced only when exposed to a magnetic field (23). Because of the puckering effect of the D-ribose, the ring slopes and skews downward toward the perimeter. Its induced magnetic field, thus, has a tangential component capable of exerting an accelerating force on the H+ as it passes by.
[(23) Morrison, R.T., & Boyd, R.N.: Organic Chemistry, 3rd Edition, p. 419 (1973)]
Each requirement for a cyclotron particle accelerator has been met on a molecular scale as follows:
(a) Oscillatory Electric Field ~ There is a continuous flow of electrons in the mitochondrion. The current oscillates as it flows through the chain of Mg++. The electric field accompanying the current oscillates in unison with it.
(b) Presence of Hydrogen Ions ~ Hydrogen ions are present in one of the compartments of the mitochondria.
(c) Auxiliary Control & Propulsion of H+ by Dipoles ~ An H+ introduced between the components of the sandwich would be attracted by an energized dipole and passed from one dipole to another around a helical circle having a diameter of approximately 30 Angstroms.
(d) Induction of Ring Current in Adenine ~ The oscillating electric field induces an oscillating ring current in the adenine ring of the molecule.
(e) Effect of Magnetic Field on Hydrogen Ion ~ As the H+ passes under the ring, it is attracted by the magnetic field accompanying the ring current and accelerated to a very high speed. The H+ soon approaches relativistic speeds because the ring current also is probably rotating at such speeds.
(f) Circular Helical Path of Hydrogen Ion ~ In a crystal having four molecules of MgATP, the path of the H+ is a circular helix. As many molecules of MgATP as are necessary would be present to accelerate the H+ and impart sufficient energy to it to penetrate the nucleus of an atom and convert it to an atom of the next higher number.
(5) General ~ There were numerous evidences that the premise upon which the study was based was true, i.e., that nuclear transmutations were indeed taking place in life organisms of all types. It was shown that there was a net loss in mass for the transmutation of Na, Km and Mn to Mg, Ca, and Fe respectively. These represented energies in the range of 8.35 MeV to 11.69 MeV. Although there was no information regarding the energy cost in effecting the transmutations, similar transmutations involving hydrogen ions were much less. Thus, there probably was a net gain in energy during the atomic transmutations.
The mitochondrion, well known as the location for energy production in the cell, was closely examined for clues to the possibility of elemental transmutations also occurring there. The presence of the ion pairs (Na, Mg), (K, Ca), and (Mn, Fe) in the mitochondrion gave strong support top such a conjecture. Moreover, the additional pair (Cu, Zn) found there but not reported by Kervran or others provided added support. These were the only ions reported to have been found in the mitochondrion.
Adenosine triphosphate (ATP) in its chelated form with Mg as MgATP has been pinpointed as the source of energy in the mitochondrion. An examination of its structure indicated that when the molecules are placed one on top of another with the Mg++ in the center, the group resembles a working model of a cyclotron on a molecular scale. When MgATP is taken apart, its components can be burned to also form energy. An analogy would be a windmill or waterwheel made of wood. Both can harness the elements to produce mechanical or electrical energy. When they are taken apart and burned, the components produce thermal energy.
The question of the primary purpose of the transmutations is of considerable interest. From Komaki's work, it would appear the transmutations were used to maintain a balance of certain elements in the human body. It is evident that health would be considerably impaired if it were attempted to obtain energy continuously from such transmutations. The body can tolerate just so much Na, K, Mg, Ca, etc., and the quantities are small.
No reports have been found of experiments which were made in which energy was measured along with elemental transmutation. Such experiments would be relatively simple to design and perform. The relatively available huge supplies of the elements which have been reported to have been transmuted and the probable large accompanying energy surplus indicate a new source of energy in the offing --- one whose supply would be unlimited.
(6) Conclusions ~ It is concluded that elemental transmutations occurring in life organisms are accompanied by losses in mass representing conversion to thermal energy and that such energy probably is a net gain when compared to the amount required to effect the transmutation.
Top ~ Home
rexresearch.com